For this case we have that the number of calories is given by the following equation:
C = mCpΔt
Where,
m: mass
Cp: specific heat
Δt : temperature differential
Substituting values we have:
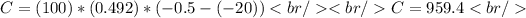
Answer:
Around 959.4 calories of heat are required to raise 100.0 g of ice from -20.0 ° c to -0.5 ° c