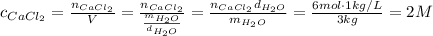Answer:
2 M
Step-by-step explanation:
Molarity is defined as the ratio of moles of a solute to the volume of a solution.
In this problem, we're dissolving calcium chloride in 3 kg of water. We may assume that calcium chloride will dissolve fully in water and won't contribute to an increase in volume of a solution, so we may approximate that the volume of this solution is equal to the volume of water.
In order to find the volume of water, we need to divide its mass by the density of water.
Since the temperature is not given, we may assume a standard density of water of
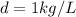 .
.
According to the molarity equation: