Answer: The proton number of the element is 21 and the nucleon number is 45
Step-by-step explanation:
We are given:
A chemical ion having symbol
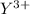
Charge on the ion = + 3
Number of electrons = 18
Number of neutrons = 24
We know that:
Charge = Number of protons - Number of electrons
Number of protons = 18 + (+3) = 21
Nucleons are defined as the sub-atomic particles which are present in the nucleus of an atom. Nucleons are protons and neutrons.
Number of nucleon in the element = 21 + 24 = 45
Hence, the proton number of the element is 21 and the nucleon number is 45