Answer : The correct option is, Na: positive; Cl: negative.
Explanation :
Ionic compound : These are the compounds where the atoms are bonded through ionic bond.
Ionic bonds are formed by the complete transfer of the electrons. The atom which looses the electron is considered as electropositive atom and the atom which gains the electron is known as electronegative atom.
The ionic bonds are formed between one metal and one non-metal.
As we know that NaCl is an ionic compound because in NaCl, the sodium cation
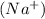 is a metal and the chloride anion
is a metal and the chloride anion
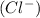 is a non-metal that means the one electron of sodium metal completely transfer to the chlorine atom and it combines to forms ionic compound through ionic bond.
is a non-metal that means the one electron of sodium metal completely transfer to the chlorine atom and it combines to forms ionic compound through ionic bond.
The sodium atom loose one electron and it is considered as electropositive atom and the chlorine atom gain one electron and it is considered as electronegative atom.
Hence, the correct option is, Na: positive; Cl: negative.