Using your knowledge of m/v = g/mL, you can start by putting that in the equation as:
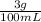
And you know that the volume is 25 mL. So you could put this into the equation as:
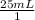
And you also know that the molar mass of KCl is 74.55g (K = 39.1 g/mol and Cl = 35.45 g/mol. Add them together to get 74.55g/mol).
We can then put this in the equation as:
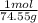
Then, we can mulitply them all together:
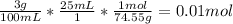
And now we know that
there are 0.01 moles of KCl in the sample.