Answer: False
Step-by-step explanation:
Kinetic energy is the energy possessed by an object by virtue of its motion.
Average kinetic energy is defined as the average of the kinetic energies of all the particles present in a system. It is determined by the equation:
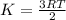
K= kinetic energy
R= gas constant
T= temperature in Kelvin
From above, it is visible that kinetic energy is directly related to the temperature of the system.