Answer: A) 1
Step-by-step explanation:
Lithium is the third element of the periodic table with atomic number of 3. The atomic number is equal to the number of electrons for a neutral atom and thus there are 3 electrons in Lithium.
The electronic configuration of Lithium (Li) is
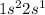 . Thus it can gain one electron to complete its octet and attain stability. Thus it can form one bond with other atoms.
. Thus it can gain one electron to complete its octet and attain stability. Thus it can form one bond with other atoms.
Example: Lithium can form one bond with flourine to form LiF by losing one electron to form
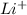 .
.