Answer: 4.2 gStep-by-step explanation:1) Radioisotope decay
formula:
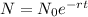
2)
Half-life formula
Replace
N = No / 2 and solve for r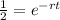
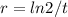
3) Replace with
t = 3.8r = ln2 / 8 = 0.1824
4) Apply the equation with No = 65g, r = 0.1824, and t = 15
N = 65g × e ^ (-0.1824 × 15) = 4.2 g
You can reasoning whether that answer makes sense:
1 half life = 3.8 days
⇒ After 3.8 days half of the mass left is 65g / 2 = 32.5 g
⇒ After other 3.8 days (a total of 7.6 days) half of 32.5 g is 16.25 g
⇒ After other 3.8 days (a total of 11.4) half of 16.25g = 8.125 g
⇒ After other 3.8 days (a total of 15.2) half of 8.125g = 4.06 g
As you see indeed 15 days is almost 4 half-lives time and so the mass remaining is close to 4.06 grams.