Answer: Option (b) is the correct answer.
Step-by-step explanation:
A chemical reaction where heat energy is absorbed by the reactant molecules is known as an endothermic reaction.
For example,
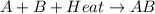
The value of
 = +ve for an endothermic reaction.
= +ve for an endothermic reaction.
In an endothermic reaction, heat being absorbed is utilized to break the bonds. Therefore, temperature of the system decreases.
On the other hand, a chemical reaction in which heat energy is released by the reactant molecules is known as an exothermic reaction.
The value of
 = -ve for an exothermic reaction.
= -ve for an exothermic reaction.
Thus, we can conclude that if the heat of reaction is negative, the reaction is exothermic.