Answer : 2.29 x 10²⁴ atoms of K are present
Explanation :
Mole and number of particles are related to each other by Avogadro's constant.
According to this, 1 mole of any substance contains 6.022 x 10²³ number of representative particles. These representative particles could be atoms, molecules, ions or formula units.
So we can say that 1 mol of K will contain 6.022 x 10²³ atoms of K.
Let us use this as a conversion factor to set up an equation.
We have been given 3.8 mol K.
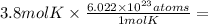 2.29 x 10²⁴ atoms of K
2.29 x 10²⁴ atoms of K
Therefore we have 2.29 x 10²⁴ atoms of K.