Answer : Partial pressure of gas A is 1.31 atm and that of gas B is 0.44 atm.
Explanation :
The partial pressure of a gas in a mixture can be calculated as
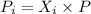
Where Pi is the partial pressure ; Xi is mole fraction and P is the total pressure of the mixture.
Therefore we have
,
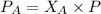
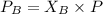
Let us find
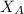 and
and
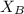
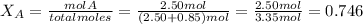
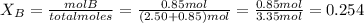
Total pressure P is given as 1.75 atm.
Let us plug in the mole fractions and P values to find partial pressures of gas A and B.
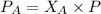
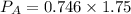
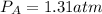
Partial pressure of gas A is 1.31 atm
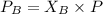
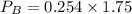
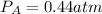
Partial pressure of gas B is 0.44 atm.
Therefore, partial pressures of gas A and B are 1.31 atm and 0.44 atm respectively.