Answer: The mass of one mole of nitrogen gas is 28 g/mol
Step-by-step explanation:
Molar mass is defined as the sum of the mass of all the atoms each multiplied its atomic masses that are present in the molecular formula of a compound. It is the mass of 1 mole of a compound or element. It is expressed in g/mol.
The chemical formula of nitrogen gas is
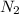
We know that:
Molar mass of nitrogen element = 14 g/mol
So, molar mass of nitrogen gas =
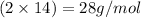
Hence, the mass of one mole of nitrogen gas is 28 g/mol