Answer:
0.0135 moles of iodine will be formed.
Step-by-step explanation:
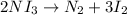
Moles of nitrogen triiodide =
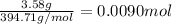
According to reaction 2 moles of nitrogen triiodide gives 3 moles of iodione gas.
Then 0.0090 mol of nitrogen triiodide will give:
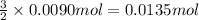
0.0135 moles of iodine will be formed.