Answer:
15 mL of 15% acid solution and 30 mL of 3% acid solution
Step-by-step explanation:
The total number of moles of the mixture
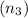 is equivalent to the addition of the number of moles of the first solution
is equivalent to the addition of the number of moles of the first solution
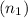 and the number of moles of the second solution
and the number of moles of the second solution
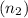 . Mathematically,
. Mathematically,

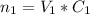
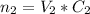
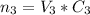
The volume of the mixture = 45 mL
The volume of first solution = x
The volume of second solution = 45 - x
Therefore:
0.15x + 0.03(45-x) = 0.07*45
0.15x + 1.35-0.03x = 3.15
0.12x = 1.8
x = 15
Thus, the volume of the first solution is 15 mL while the volume of the second solution is 30 mL.