Answer:
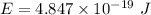 is the difference in the energy levels.
is the difference in the energy levels.
Step-by-step explanation:
Given:
- wavelength of the light emitted for transition of energy levels,
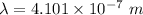
- initial energy level,
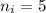
- final energy level,

Now as we know that energy can be given by:
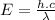
where:
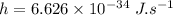 Planck's Constant
Planck's Constant
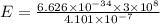
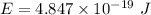 is the difference in the energy levels.
is the difference in the energy levels.