Answer:
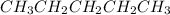
Explanation: Hydrocarbons are the compounds which contain only carbon and hydrogen as their constituent elements. These are considered to be as the non polar species as because of the less electronegativity difference between the two components.
Now as it is a non polar species thus it will dissolve only a non polar solute following the principle of 'Like dissolves Like'. Thus the most non polar species given in the options is pentane. Cyclohexane will definitely dissolve this pentane as both are non polar in nature.
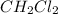 , HI and NaBr are polar specie which cannot be dissolved in non polar species.
, HI and NaBr are polar specie which cannot be dissolved in non polar species.