To solve this problem we will find the proportion in terms of the solution and solution. According to the data given, the amount of solute is 1,929g in a solution of 25,725g, therefore the mass / mass percentage would be:
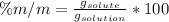
Replacing,
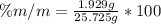
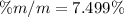
Therefore there is 7.499% of solute in the urine sample.