Answer:
N₂= 697.24 m/s,
O₂= 652.38 m/s
CO₂= 393.35 m/s
Step-by-step explanation:
The rms speed,
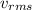 of a gas is the following:
of a gas is the following:
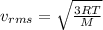
where R: is the ideal gas constant, T: is the temperature and M: is the molar mass.
Knowing that the molar mass of the given gases are:
N₂ = 14.0067 g/mol = 14.0067x10⁻³ kg/mol
O₂ = 15.999 g/mol = 15.999x10⁻³ kg/mol
CO₂ = 44.009 g/mol = 44.009x10⁻³ kg/mol
Also that T is 0 °C = 273 K, and R = 8.314 J/ K.mol = 8.314 kg.m². s⁻². K⁻¹. mol⁻¹. The rms speed of the gases are:
For N₂:
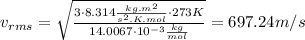
For O₂:
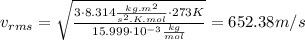
For CO₂:
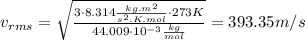
Therefore, the rms speed of N₂ is 697.24 m/s, of O₂ is 652.38 m/s and of CO₂ is 393.35 m/s
I hope it helps you!