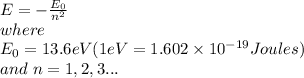Answer:
The energies corresponding to each of the allowed orbitals are called energy levels.
Step-by-step explanation:
A scientist known as Niels Bohr put forward that electrons in an atom covers some permitted orbitals with a specific energy. In other words, the energy of an electron in an atom is not continuous, but 'quantized.' The energies corresponding to each of the allowed orbitals are called energy levels.