Answer:

Step-by-step explanation:
To convert from moles to mass, we need to use the molar mass of the element. This can be found on the Periodic Table. It tells us the grams of magnesium in 1 mole of magnesium.
- Magnesium (Mg): 24.305 g/mol
Use the molar mass as a fraction or ratio.
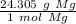
Multiply by the given number of moles (5.05)
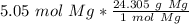
The moles of magnesium will cancel each other out.
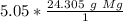
The denominator of 1 can be ignored to create a simple multiplication problem.
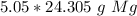
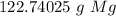
The original measurement (5.05 moles) has 3 significant figures. Therefore we must round our answer to the 3 sig figs. For the number we calculated, that is the ones place.
The 7 in the tenth place tells us to round the 2 to a 3.
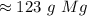
There are about 123 grams of magnesium in 5.05 moles.