Answer:
The final temperature is:- 7428571463.57 °C
Step-by-step explanation:
The expression for the calculation of heat is shown below as:-
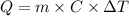
Where,
 is the heat absorbed/released
is the heat absorbed/released
m is the mass
C is the specific heat capacity
 is the temperature change
is the temperature change
Thus, given that:-
Mass of water = 1.75 mg = 0.00175 g ( 1 g = 0.001 mg)
Specific heat of water = 4.18 J/g°C
Initial temperature = 35 °C
Final temperature = x °C
![\Delta T=(x-35)\ ^0C/tex] </p><p>Q = [tex]1.3* 10^4](https://img.qammunity.org/2020/formulas/chemistry/high-school/t1s4qmk8swu6r4gmhtsoomyoffzu94au2h.png) kcal
kcal
Also, 1 kcal = 4.18 kJ =
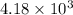 J
J
So, Q =
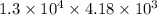 J = 54340000 J
J = 54340000 J
So,
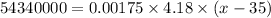
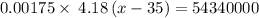

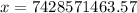
Thus, the final temperature is:- 7428571463.57 °C