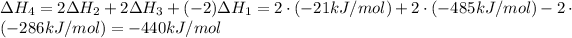Answer:
a. Lithium is in its standard state
b.
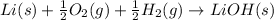
c.
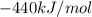
Step-by-step explanation:
a. Elements in their standard states at room temperature and 1 atm pressure would have an enthalpy of formation of 0 kJ/mol. Lithium is metal at standard conditions, so its enthalpy of formation is 0 kJ/mol.
b. The equation representing the formation of a compound should following the rules below:
- strictly 1 mol of the product formed;
- all reactants in their standard states at room temperature and 1 atm pressure.
We should, hence, form 1 mol of LiOH from the following species:
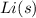 : solid lithium metal in its standard state;
: solid lithium metal in its standard state;
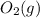 : oxygen gas as diatomic in its standard state;
: oxygen gas as diatomic in its standard state;
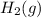 : hydrogen gas as diatomic in its standard state.
: hydrogen gas as diatomic in its standard state.
We obtain the following equation:
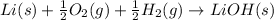
c. Firstly, write the equation for the enthalpy of formation of water using the guidelines in (b):
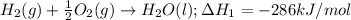
Now given the equation:
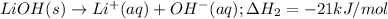
As well as:
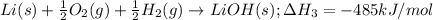
Notice that multiplying reaction (2) by 2, multiplying reaction (3) by 2, multiplying reaction (1) by -2 (that is, multiplying by 2 and reversing it) and adding them together will yield the target equation:
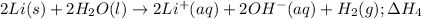
According to Hess's Law, we will perform the same steps with the enthalpy values and we will add them to get the final enthalpy value: