Answer:
W = 7.214 k J
Step-by-step explanation:
given,
time of operation = 5 minutes
mass of ice melt = 5.90 × 10⁻² kg
L_f = 3.34 × 10⁵ J/kg
Work done = ?
We know work done
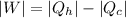
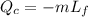
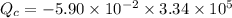
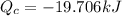
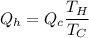
T_H is the heat from hot reservoir = 373 K
T_C is the energy released to cold reservoir = 273 K
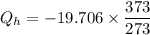
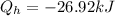
now,
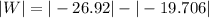
W = 7.214 k J