Four ions result from the dissociation of one molecule of
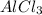
Answer: Option A
Step-by-step explanation:
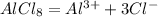
Here, it is understood that when one-mole aluminum chloride salt dissociates than it gives one mole of aluminum ion and three moles of the chloride ion here.
This process is understood by learning the stoichiometry of chemical equations where the number of mole of reactant should be equal to the number of mole of products for any balanced chemical equation. Balanced chemical equations give quantitative data by providing a measure of elements.