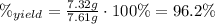Answer:
(a) HBr;
(b) 7.61 g;
(c) 96.2 %
Step-by-step explanation:
Firstly, write the balanced chemical equation:
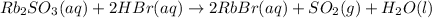
(a) Find moles of each reactant dividing the mass by the molar mass of rubidium sulfite, then applying the ideal gas law for HBr:
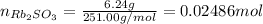
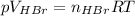
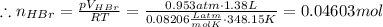
Find the limiting reactant by dividing each moles by the stoichiometric coefficients and comparing the two numbers:
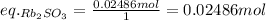
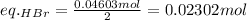
That said, the equivalent of HBr is lower, so it's the limiting reactant.
(b) According to the balanced equation, the moles of HBr are equal to the moles of RbBr, so moles of RbBr theoretically are equal to:
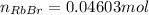
Using the molar mass of RbBr, convert this into mass:
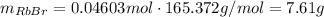
(c) To find the percent yield, divide the actual mass produced by the theoretical mass calculated in (b) and multiply by 100 %: