Answer:
The mass of radon that decompose = 63. 4 g
Step-by-step explanation:
R.R = P.E/(2ᵇ/ⁿ)
Where R.R = radioactive remain, P.E = parent element, b = Time, n = half life.
Where P.E = 100 g , b = 5.55 days, n = 3.823 days.
∴ R.R = 100/
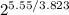
R.R = 100/
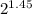
R.R = 100/2.73
R.R = 36.63 g.
The mass of radon that decompose = Initial mass of radon - Remaining mass of radon after radioactivity.
Mass of radon that decompose = 100 - 36.63
= 63.37 ≈ 63.4 g
The mass of radon that decompose = 63. 4 g