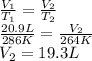Answer:
19.3 L
Step-by-step explanation:
Initially, Helium gas occupies a volume V₁ = 20.9 L at a temperature T₁ = 13°C = 286 K. Then, it occupies a volume V₂ at a temperature T₂ = -9°C = 264 K. We can find the new volume using Charles' law (assuming constant pressure and ideal gas behavior).