Answer:
d. When aluminum-28 undergoes beta decay, silicon-28 is produced.
Explanation:
- aluminum-27 has an abundance percentage >99.9%. aluminum-28 will have less than that.
so, option a is wrong.
- Aluminum-28 has one more neutron than aluminum-27.
- Aluminum-28 will have 13 electrons, 13 electrons and 15 neutrons.
- Aluminum-27 will have 13 electrons, 13 electrons and 14 neutrons.
so,option b is wrong.
beta decay reaction for aluminum-28 is :
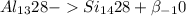
[where,
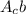 represents, element A,
represents, element A,
b: its mass number[=number of protons+number of neutrons in the element]
, c : atomic number[=number of protons in the element]
so, option c is wrong and option d is correct.