Answer:
68 grams
Step-by-step explanation:
Reaction that produces ammonia when nitrogen and hydrogen reacts is given by :-
 + 3
+ 3
 → 2N
→ 2N
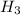
Clearly ,
When one mole of nitrogen reacts , two moles of ammonia will be produced.
Now,
weight of 1 mole of the Nitrogen = 28.0 grams
In 56.0 grams of the nitrogen , two moles will be present.
Thus , 4 moles of ammonia will be produced .
Weight of one mole of nitrogen = 17 grams.
Thus a total of 17× 4 = 68 grams f nitrogen will be produced.