Step-by-step explanation:
It is known that efficiency is denoted by
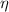 .
.
The given data is as follows.
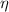 = 0.82,
= 0.82,
 = (21 + 273) K = 294 K
= (21 + 273) K = 294 K
 = 200 kPa,
= 200 kPa,
 = 1000 kPa
= 1000 kPa
Therefore, calculate the final temperature as follows.
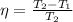
0.82 =
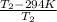
 = 1633 K
= 1633 K
Final temperature in degree celsius =

=
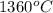
Now, we will calculate the entropy as follows.
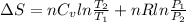
For 1 mole,
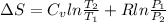
It is known that for
 the value of
the value of
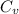 = 0.028 kJ/mol.
= 0.028 kJ/mol.
Therefore, putting the given values into the above formula as follows.
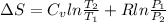
=

= 0.0346 kJ/mol
or, = 34.6 J/mol (as 1 kJ = 1000 J)
Therefore, entropy change of ammonia is 34.6 J/mol.