Answer:
MgO
Step-by-step explanation:
From the table, Mg atom has 2 valence electrons while oxygen atom has a deficit of 2 valence electrons.
When the two atoms react together, the 2 valence electrons from the Mg atom are donated to compensate for the 2 deficit electrons in the oxygen atom.
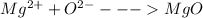
Hence, one atom of Mg is required to react with one atom of oxygen to produce MgO with each atom having stable shells.