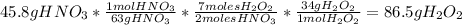Answer:
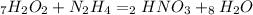 (H = 1; N = 14; O = 16) 1. How many moles of
(H = 1; N = 14; O = 16) 1. How many moles of
 are formed from 0.025 moles of
are formed from 0.025 moles of
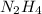 ? 2. How many moles of
? 2. How many moles of
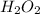 are needed if 1.35 moles of
are needed if 1.35 moles of
 are formed? 3. How many grams of
are formed? 3. How many grams of
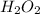 are needed to produce 45, 8 g of
are needed to produce 45, 8 g of
 ?
?
1. 0.05 moles of

2. 1.18 moles of
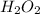
3. 86.5g of
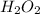
Step-by-step explanation:
First write the balanced chemical equation given by the problem:
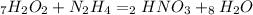
1. Calculate how many moles of
 are formed from 0.025 moles of
are formed from 0.025 moles of
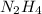 using the reaction stoichiometry:
using the reaction stoichiometry:
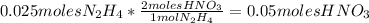
2. Calculate how many moles of
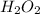 are needed if 1.35 moles of
are needed if 1.35 moles of
 are formed using the reaction stoichiometry:
are formed using the reaction stoichiometry:
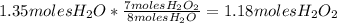
3. Calculate how many grams of
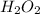 are needed to produce 45,8 g of
are needed to produce 45,8 g of
 :
:
- Calculate the molar mass of the
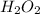 :
:
Molar mass H: 1

Molar mass O: 16

Molar mass
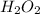 =(2*1
=(2*1
 )+(2* 16
)+(2* 16
 )=34
)=34

- Calculate the molar mass of the
 :
:
Molar mass H: 1

Molar mass N: 14

Molar mass O: 16

Molar mass
 =(1*1
=(1*1
 )+(1*14
)+(1*14
 )+(3*16
)+(3*16
 )=63
)=63