Answer:
The atomic weight of the "y" compound is 80.
Step-by-step explanation:
The carbon atomic weight = 12 g/mol
Atomic weight of "X" =
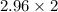
One mole of "X" element has mass 0.444 times the mass of one mole of element "Y".
So, atomic weight of "X" element =
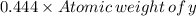
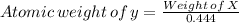
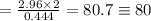
Therefore, The atomic weight of the "y" compound is 80.