Answer:
2KI + Cl₂ → 2KCl + I₂
Step-by-step explanation:
The reaction equation is given as:
KI + Cl₂ → KCl + I₂
The problem at hand is to balance this chemical reaction. To solve this problem we use a mathematical approach;
aKI + bCl₂ → cKCl + dI₂
Conserving K : a = c
I : a = 2d
Cl : 2b = c
Now let a = 1, c = 1 , d =
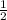 , b =
, b =
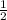 , ;
, ;
Multiply through by 2;
a = 2, b = 1 , c = 2, d = 1
2KI + Cl₂ → 2KCl + I₂