Answer: The amount of heat released when 0.211 moles of
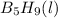 reacts is 554.8 kJ
reacts is 554.8 kJ
Step-by-step explanation:
The chemical equation for the reaction of
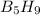 with oxygen gas follows:
with oxygen gas follows:
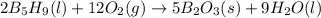
The equation for the enthalpy change of the above reaction is:
![\Delta H_(rxn)=[(5* \Delta H_f_((B_2O_3(s))))+(9* \Delta H_f_((H_2O(l))))]-[(2* \Delta H_f_((B_5H_9(l))))+(12* \Delta H_f_((O_2(g))))]](https://img.qammunity.org/2020/formulas/chemistry/college/er0xvsfanmzxhm4hn4ap6xuavivf2emamb.png)
We are given:
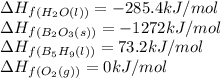
Putting values in above equation, we get:
![\Delta H_(rxn)=[(2* (-1272))+(9* (-285.4))]-[(2* (73.2))+(12* (0))]\\\\\Delta H_(rxn)=-5259kJ](https://img.qammunity.org/2020/formulas/chemistry/college/aevq3fse7o7ljgrvz4op7ndf3oj5khl1sz.png)
To calculate the amount of heat released for the given amount of
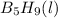 , we use unitary method, we get:
, we use unitary method, we get:
When 2 moles of
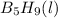 reacts, the amount of heat released is 5259 kJ
reacts, the amount of heat released is 5259 kJ
So, when 0.211 moles of
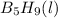 will react, the amount of heat released will be =
will react, the amount of heat released will be =
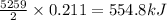
Hence, the amount of heat released when 0.211 moles of
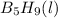 reacts is 554.8 kJ
reacts is 554.8 kJ