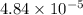Answer: The value of
 is
is
Step-by-step explanation:
We are given:
Initial moles of ammonia = 0.0280 moles
Initial moles of oxygen gas = 0.0120 moles
Volume of the container = 1.00 L
Concentration of a substance is calculated by:
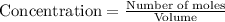
So, concentration of ammonia =
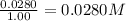
Concentration of oxygen gas =
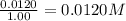
The given chemical equation follows:
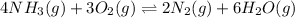
Initial: 0.0280 0.0120
At eqllm: 0.0280-4x 0.0120-3x 2x 6x
We are given:
Equilibrium concentration of nitrogen gas =
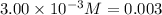
Evaluating the value of 'x', we get:
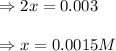
Now, equilibrium concentration of ammonia =
![0.0280-4x=[0.0280-(4* 0.0015)]=0.022M](https://img.qammunity.org/2020/formulas/chemistry/college/egfd0ku4lv0cealc234pddjhmk9gr5l3os.png)
Equilibrium concentration of oxygen gas =
![0.0120-3x=[0.0120-(3* 0.0015)]=0.0075M](https://img.qammunity.org/2020/formulas/chemistry/college/6vqtb5o4vgifqk6kwu6ngdz1mwsn4suurr.png)
Equilibrium concentration of water =
![6x=(6* 0.0015)]=0.009M](https://img.qammunity.org/2020/formulas/chemistry/college/4hvw7g0yztywaxmroedmvp4c8358tgy619.png)
The expression of
 for the above reaction follows:
for the above reaction follows:
![K_(eq)=([H_2O]^6* [N_2]^2)/([NH_3]^4* [O_2]^3)](https://img.qammunity.org/2020/formulas/chemistry/college/u49253cwius4ds8g8wh70ygls3msy13zjt.png)
Putting values in above expression, we get:
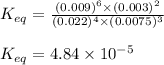
Hence, the value of
 is
is