Answer:
B. use light of a shorter wavelength.
Step-by-step explanation:
We know that
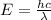
h= plank's constant
c= speed of light
λ= wavelength of the incident light
so, in order to have sufficient energy for for the emission of electron, the incident's radiation must have λ small enough.
B. use light of a shorter wavelength.