Answer:
A) calcium-0 B) gallium-1 C) silicon-2 D) vanadium-3 E) carbon-2 F) neon-0
Step-by-step explanation:
Hund's rule of maximum multiplicity:-
Firstly, every orbital which is present in the sublevel is singly occupied and then the orbital is doubly occupied.
(A) Calcium.
The electronic configuration is -
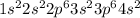
Thus, 4s orbital is fully filled and thus there is no singly filled orbital in calcium.
(B) Gallium.
The electronic configuration is -

Thus, 4s orbital is fully filled and p orbital can singly filled 3 electrons. Thus, no electrons will be paired and thus, 1 orbital will be singly filled in gallium.
(C) Silicon.
The electronic configuration is -

Thus, 3s orbital is fully filled and p orbital can singly filled 3 electrons. Thus, no electrons will be paired and thus, 2 orbitals will be singly filled in silicon.
D) Vanadium
The electronic configuration is -

Thus, 4s orbital is fully filled and d orbital can singly filled 5 electrons. Thus, 3 orbitals will be singly filled in vanadium.
(E) Carbon.
The electronic configuration is -
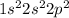
Thus, 2s orbital is fully filled and p orbital can singly filled 3 electrons. Thus, Carbon has 2 singly occupied orbitals.
F) Neon
The electronic configuration is -
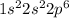
Thus, 2s orbital is fully filled and p orbital can singly filled 3 electrons which is also fully filled. So, there is no singly filled orbital in neon.