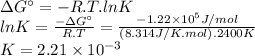Answer:
K = 2.21 × 10⁻³
Step-by-step explanation:
The Gibbs free energy change (ΔG°) at a temperature T = 2400 K is 1.22 × 10⁵ J/mol. We can calculate the equilibrium constant (K) using the following expression.
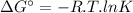
where,
R is the ideal gas constant (8.314 J/mol.K)
T is the absolute temperature