Answer : The correct options are, (a) and (d)
Explanation :
The given reversible reaction is:
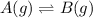
Equilibrium constant : It is defined as the equilibrium constant. It is defined as the ratio of concentration of products to the concentration of reactants.
The equilibrium constant expression for the given reaction will be:
![K=([B])/([A])](https://img.qammunity.org/2020/formulas/chemistry/college/j0fc2psaj1tut709unmet47fmnfsb1chry.png)
As per question, when the value of equilibrium constant is greater than 1 then concentration of B is greater than concentration of A at equilibrium.
In the given options, option (a) and option (d) K values would indicate that there is more B than A at equilibrium because value of equilibrium constant is greater than 1.
Hence, the correct options are, (a) and (d)