Answer:
The isomer is 2,2-dimethylpropane
Step-by-step explanation:
The three constitutional isomers of pentane(
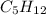 ) are:
) are:
1. n-pentane
2. 2-methylbutane
3. 2,2-dimethylpropane
After chlorination of n-pentane we can get three different products(excluding optical isomers) depending on the position of chlorine atom.
After chlorination of 2-methylbutane we get four different products(excluding optical isomers).
But in the case of 2,2-dimethylpropane we get only one product as all the four carbon atoms attached to a central carbon atom are identical.
This explanation becomes clear after seeing the figure below.