Answer:
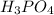
Step-by-step explanation:
We start this question by assuming we have 100 g of phosphoric acid. Based on the percentages, this means 3.1 g of it is hydrogen, 31.6 g of it is phosphorus and 65.3 of it is oxygen. Now we can calculate the empirical formula
H : 3.1/1.008 = 3.075 ≈ 3
P : 31.6/30.97 = 1.020 ≈ 1
O : 65.3/15.99 = 4.08 ≈ 4
This would mean that its formula is H3PO4 which has a relative molecular mass of 3 (1.008) + 1(30.97) + 4(15.99) = 97.954. Since the empirical molecular mass is equal to the molar mass of this substance in grams, then we have found the molecular mass