Answer:
The pH of the solution is 3.0
Step-by-step explanation:
Considering:

Or,
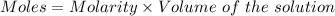
Given :
For
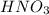 :
:
Molarity = 0.0030 M
Volume = 100 mL
The conversion of mL to L is shown below:
1 mL = 10⁻³ L
Thus, volume = 100×10⁻³ L
Thus, moles of
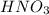 :
:
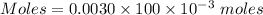
Moles of
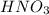 = 0.0003 moles
= 0.0003 moles
Volume added = 200 mL
Total volume = 200 + 100 mL = 300 mL = 0.3 L
Thus,
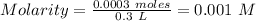
SInce, it is strong acid. Thus, [H⁺] = 0.001 M
pH is defined as the negative logarithm of the concentration of hydrogen ions.
Thus,
pH = - log [H⁺] = - log 0.001 = 3.0
The pH of the solution is 3.0