Answer:
The volume occupied by 2.18 moles of
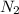 is 48.832L
is 48.832L
Step-by-step explanation:
The volume occupied by 1 mole of
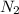 is 22.4 L
is 22.4 L
The volume occupied by 1 mole of any gas is 22.4 L
The number of moles of
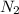 = 2.18 moles
= 2.18 moles
The volume occupied by 2.18 moles of
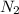 :
:

Hence, the volume occupied by 2.18 moles of
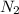 is 48.832L
is 48.832L