Answer:
Quantity of Carbon is 4.09 gm
Step-by-step explanation:
Equation of carbon reacting with oxygen to give carbon dioxide is given by
C +
 ⇒ C
⇒ C

One mole of carbon reacts with one mole of Oxygen in this reaction to give One mole of Carbon dioxide.
So, 12 gm of carbon reacts with 32 gm of Oxygen in this reaction to give 44 gm of carbon dioxide.
15 gm of C
 was formed in this reaction
was formed in this reaction
Oxygen used in this reaction =
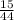 ×32 = 10.91 gm ,
×32 = 10.91 gm ,
Thus Oxygen is in sufficient quantity in the reaction.
Now,
Carbon that must be used =
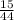 ×12 = 4.09 gm.
×12 = 4.09 gm.