Answer:
The correct answer is:
The pH of pure water decreases as the temperature is increased.
Step-by-step explanation:
Ionization constant of water :
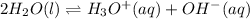
Water will dissociate into equal amount of hydronium and hydroxide ions
![K_w=[H_3O^+]* [OH^-]](https://img.qammunity.org/2020/formulas/chemistry/college/of30j4riha7y9a6ia5fn9lkke8etef2txl.png)
As we can see that from the given information that ionic product of water increase with increase in temperature which means concentration of hydronium ion concentration increases.
And according to definition of pH of the solution is a negative logarithm of hydronium ions concentration.
![pH=-\log [H_3O^+]](https://img.qammunity.org/2020/formulas/chemistry/middle-school/xj6fwrpeduepfcp6k4cv1s827uf5c0pbp1.png)
The pH of the solution of solution is inversely proportional to the concentration of hydronium ions.
As the hydronium ion concentration of water increases with increase in temperature , the pH of the water will decrease.