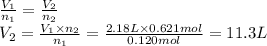Answer:
11.3 L
Step-by-step explanation:
Initially, the cylinder had n₁ = 0.120 mol of gas in an initial volume V₁ = 2.18 L. At the end, it had n₂ = 0.621 mol in an unknown volume V₂. According to the Avogadro's law, in the same conditions of pressure and temperature, the volume is directly proportional to the number of moles.