Answer:
a. The phenolphthalein acts as a color changing indicator to signal the endpoint of the reaction.
Step-by-step explanation:
Phenolphthalein is an organic substance with chemical formula
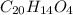 .
.
It is a substance commonly used in acid-base titrations to indicate the end point in the titration because phenolphthalein is colorless in acidic solutions but turns a purplish-pink color in basic solutions.
In this way it helps visually to notice when the final point of the titration has been reached.