Answer:
The answer to your question is 92.7%
Step-by-step explanation:
Balanced Chemical reaction
3 Zn + Fe₂(SO₄)₃ ⇒ 2Fe + 3ZnSO₄
Molecular weight
Zinc = 65.4 x 3 = 196.2g
Iron (III) = 56 x 2 = 112 g
Proportions
196.2 g of Zinc ------------------ 112 g of Iron
20.4 g of Zinc ----------------- x
x = (20.4 x 112) / 196.2
x = 2284.8/196.2
x = 11.65 g of Iron
% yield =
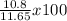
% yield = 0.927 x 100
% yield = 92.7