Answer:
1. False
2. False
3. True
Step-by-step explanation:
Identify all statements are true
1. zeroth order rate constants have units of inverse seconds. FALSE.
The rate law for a zeroth order rate reaction has the following form:
r = k
where,
r is the rate of the reaction
k is the rate constant
k has the same units than r, that is, M . s⁻¹.
2. first order reactions have half lives that are dependent on time . FALSE.
Half-life (t1/2) of a first order reaction can be calculated using the following expression.
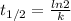
As we can see, half-life does not depend on time.
3. catalysts cannot appear in the rate law for a reaction. TRUE.
The rate law has the following form:
r = k . [A]ᵃ . [B]ᵇ
where,
[A] and [B] are the concentrations of the reactants
a and b are the reaction orders
Catalysts do not appear in the rate law.